| PRESS RELEASE |
| Source: Tokyo Institute of TechnologyNagoya Institute of TechnologyNational Institute of Technology, Niihama CollegeThe Comprehensive Research Organization for Science and SocietyJapan Atomic Energy Agency |
| For immediate release: May 16, 2018 |
|
| Apatite-Type Materials without Interstitial Oxygens Show High Oxide-Ion Conductivity by Overbonding |
(Tokyo, May 16) Scientists at Tokyo Institute of Technology; Nagoya Institute of Technology; National Institute of Technology, Niihama College; Neutron Science and Technology Center, The Comprehensive Research Organization for Science and Society (CROSS); and Japan Atomic Energy Agency have shown the overbonding of channel oxygens in La-rich apatite-type lanthanum silicates, rather than the presence of the interstitial oxygens, to be responsible for the high oxide-ion conductivity. This concept of "high oxide-ion conductivity by overbonding" opens the door for designing better ion conductors, which could be useful in energy conversion and environmental protection. |
Solid oxide electrolytes have been extensively studied due to their wide range of applications in solid oxide fuel cells (SOFCs), oxygen membranes, catalysts, and gas sensors. Electrolytes with high oxide-ion conductivity at temperatures below 600℃ are required to decrease the operation temperature of SOFCs. Professor Susumu Nakayama at National Institute of Technology, Niihama College has discovered in 1995 the extremely high oxide-ion conductivity in the intermediate temperature range below 600℃, which has encouraged many researchers to study the structural origin of this phenomenon. |
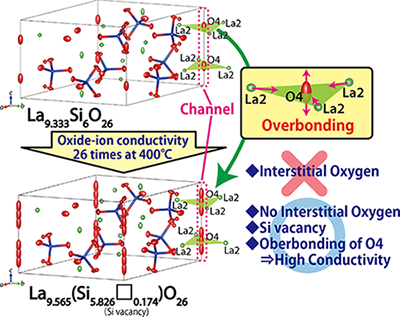
It was believed that the high oxide-ion conductivity of apatite-type materials is due to interstitial oxygens. However, in this novel study, Professor Masatomo Yashima, Dr. Kotaro Fujii at Tokyo Institute of Technology (Tokyo Tech), and their colleagues have shown that apatite-type materials contain Si vacancies, but not oxygen interstitials. The Si vacancies in the materials have originally been proposed by Professor Koichiro Fukuda at Nagoya Institute of Technology. |
Through single-crystal neutron diffraction studies using the SENJU diffractometer installed at MLF, J-PARC facility (Figure 1), they were able to accurately determine the crystal structures of the apatite materials La9.333Si6O26 and La-rich La9.565 (Si5.826□0.174) O26 (□ denotes Si vacancy) including occupancy factors, atomic displacement parameters, and spatial distributions of oxygen atoms. They also measured the density and oxide-ion conductivity of the two materials. In this work, La9.565 (Si5.826□0.174) O26 was selected because of its high oxide-ion conductivity. |
By structure analyses using the diffraction data, the researchers found Si vacancies, no interstitial oxygens, and larger positional disorder of the oxide ion at the O4 site in the apatite channel as compared to the basic material La9.333Si6O26 (Figure 2) . The lower activation energy for the oxide-ion conduction along the c axis was found to be the main reason for the higher oxide-ion conductivity of La9.565 (Si5.826□0.174) O26 as compared to La9.333Si6O26. The excess La yielded the overbonding of the O4 oxide ion in La9.565 (Si5.826□0.174) O26 as compared with La9.333Si6O26, which led to higher oxide-ion mobility and conductivity of La9.565 (Si5.826□0.174) O26 with Si vacancies (Figure 2). Density measurements by the Archimedes method supported the presence of Si vacancies in La9.565 (Si5.826□0.174) O26. |
Thus, the researchers proposed that excess La cations are responsible for overbonded channel oxygens along the c axis, which leads to highly anisotropic atomic displacement and high oxygen mobility. Hence this novel concept of "high oxide-ion conductivity by overbonding" may be useful for designing better ion conductors. |
 【 Reference 】 【 Reference 】 
|
Authors : Kotaro Fujii,1 Masatomo Yashima,1,* Keisuke Hibino,1 Masahiro Shiraiwa,1 Koichiro Fukuda,2 Susumu Nakayama,3 Nobuo Ishizawa,4 Takayasu Hanashima5 and Takashi Ohhara6 |
Title of original paper : High oxide-ion conductivity by the overbonded channel oxygens in Si-deficient La9.656 (Si5.826□0.174) O26 apatite without interstitial oxygens |
Journal : Journal of Materials Chemistry A |
DOI : 10:1039/C8TA02237B |
Affiliations : 1. Department of Chemistry, School of Science, Tokyo Institute of Technology, Tokyo 152-8551, Japan 2. Department of Life Science and Applied Chemistry, Graduate School of Engineering, Nagoya Institute of Technology, Nagoya 466-8555, Japan 3. Department of Applied Chemistry and Biotechnology, National Institute of Technology, Niihama College, Niihama 792-8580, Japan 4. Advanced Ceramics Research Center, Nagoya Institute of Technology, Tajimi, 507-0033, Japan 5. Neutron Science and Technology Center, CROSS, Tokai, Ibaraki 319-1106, Japan 6. J-PARC Center, Japan Atomic Energy Agency, Tokai, Ibaraki 319-1195, Japan |
*Corresponding author's name and email : Professor Masatomo Yashima yashima  cms.titech.ac.jp |
 【 Figure 】 【 Figure 】 
|
| |
 * Click here to enlarge. * Click here to enlarge. |
| Figure 1 : (a) A schematic figure and (b) a photograph of the SENJU diffractometer installed at the J-PARC facility. (c) Measured single-crystal neutron diffraction images. |
| |
 * Click here to enlarge. * Click here to enlarge. |
| Figure 2 : Crystal structures of the apatite-type oxide-ion conductors La9.333Si6O26 and La9.565 (Si5.826□0.174) O26 determined from the single-crystal neutron diffraction studies. |
| |
 【 Contact 】 【 Contact 】 
|
Emiko Kawaguchi Public Relations Section, Tokyo Institute of Technology Email: media  jim.titech.ac.jp +81-3-5734-2975 |
 【 About Tokyo Institute of Technology 】 【 About Tokyo Institute of Technology 】 
|
Emiko Kawaguchi Tokyo Institute of Technology stands at the forefront of research and higher education as the leading university for science and technology in Japan. Tokyo Tech researchers excel in a variety of fields, such as material science, biology, computer science and physics. Founded in 1881, Tokyo Tech has grown to host 10,000 undergraduate and graduate students who become principled leaders of their fields and some of the most sought-after scientists and engineers at top companies. Embodying the Japanese philosophy of "monotsukuri," meaning technical ingenuity and innovation, the Tokyo Tech community strives to make significant contributions to society through high-impact research. |
|
©2018 J-PARC Center. All rights reserved. |

