Getting through the bottleneck- A new class of layered perovskite with high oxygen-ion conductivity -
Scientists at the Tokyo Institute of Technology (Tokyo Tech) have discovered a layered perovskite that shows unusually high oxide-ion conductivity, based on a new screening method and a new design concept. Such materials are hard to come by, so the discovery of this material, the new method and design concept will make the realization of many environment-friendly technologies.
Upon hearing the word "conductor" in reference to chemistry, most will immediately think of the movement of electrons within a material. But electrons are not the only particles that can move across a material; oxide ions can too. Many materials scientists and engineers are currently searching for materials with high oxide-ion conductivity. Such materials have many potential applications, particularly in the development of environmentally friendly technologies. For example, oxide-ion conductors could be used in fuel cells, which directly convert clean fuel such as hydrogen into electrical energy, or in oxygen separation membranes, which could be useful in systems for capturing the CO2 we produce by burning coal or fossil fuels.
Unfortunately, high oxide-ion conductivities can be achieved by a limited number of structure families of materials. The perovskites are one such structure family. Perovskites and layered perovskites have special crystal structures that sometimes exhibit outstanding physical and chemical properties. Prof. Masatomo Yashima and colleagues from the Tokyo Institute of Technology studied a class of layered perovskites, a Dion-Jacobson phase, where two-dimensional perovskite-like "slabs" are stacked and separated by a layer of alkali metal ions, such as the cesium cation (Cs+). In their paper published in Nature Communications, Professor Yashima and his colleagues explain their motivation: "Numerous studies have been conducted on the electrical properties of Dion-Jacobson phases, such as their proton, lithium-ion and sodium-ion conduction. However, there are no reports on the oxide-ion conduction in Dion-Jacobson phases."
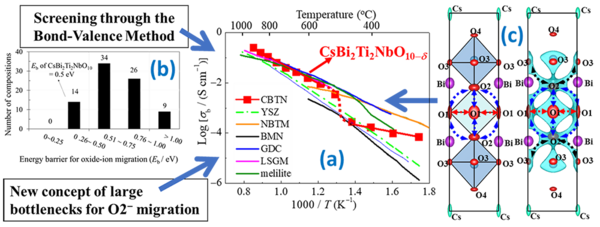
In their study, the scientists first screened sixty-nine potential Dion-Jacobson phases using the bond-valence method. This method allowed them to calculate the energy barriers for oxide-ion migration in each Dion-Jacobson phase, from which they identified CsBi2Ti2NbO10−δ (CBTN) as a promising candidate because it has a low energy barrier and does not contain expensive rare-earth elements. Further, they prepared CBTN samples and found that the oxide-ion conductivity of CBTN was higher than those of many other oxide-ion conductors, such as the conventional yttria-stabilized zirconia (Figure 1).
To understand what causes such high oxide-ion conductivity in CBTN, the scientists analyzed its crystal structure and watched how the structure changes with temperature. Using a super-high-resolution neutron diffractometer, SuperHRPD at J-PARC, they then identified several possible paths across the crystal lattice through which oxide ions could migrate at high temperatures (Figure 1c). Most importantly, they discovered a novel mechanism that seems to be one of the causes of the high oxide-ion conductivity: Rise in temperature makes oxygen vacancies appear, which facilitate oxide-ion migration. The large Cs cations and the displacement of the Bi ions in the structure at high temperatures expand the bottlenecks, enabling oxide-ion migration.
This study paves the way for finding inexpensive novel oxide-ion conductors. Based on this oxide-ion conduction mechanism, one can enhance the oxide-ion conductivities of materials of the CBTN family by modifying the chemical composition of CBTN through the addition of impurities (doping). "The present findings of high oxide-ion conductivity in this new structure family, the Dion-Jacobson-type CsBi2Ti2NbO10−δ, and the new enlarged bottleneck concept introduced, could facilitate the design of novel oxide-ion conductors based on Dion-Jacobson phases," Prof. Yashima and his colleagues conclude. The findings of this study open up the possibilities for many novel applications that will lead to a sustainable future. In fact, the present work was chosen as Editors' Highlights of Nature Communications.
Figure 1. The mechanism, design concept, and structural characteristics that afford high oxide-ion conductivity in CsBi2Ti2NbO10−δ
(a) The oxide-ion conductivity of CsBi2Ti2NbO10−δ is higher than those of many materials reported previously. (b) By screening sixty-nine potential materials using the bond-valence method, CsBi2Ti2NbO10−δ was selected. A new design concept for its high oxide-ion conductivity is proposed: enlarged bottlenecks. (c) Structure of CsBi2Ti2NbO10−δ (left) and the bond-valence-based energy landscape for an oxide ion at 0.6 eV (right), which marks all the possible oxide-ion migration. The bottlenecks for oxide-ion migration are enlarged in CsBi2Ti2NbO10−δ.
Reference
| Authors | Wenrui Zhang1, Kotaro Fujii1, Eiki Niwa1, Masato Hagihala2,3, Takashi Kamiyama2,3 and Masatomo Yashima*1 |
|---|---|
| Title of original paper | Oxide-ion conduction in the Dion-Jacobson phase CsBi2Ti2NbO10−δ |
| Journal | Nature Communications |
| DOI | https://doi.org/10.1038/s41467-020-15043-z |
| Affiliations | 1Department of Chemistry, School of Science, Tokyo Institute of Technology 2Institute of Materials Structure Science, High Energy Accelerator Research Organization (KEK) 3Japan Proton Accelerator Research Complex (J-PARC) |
*Corresponding author's email: yashima[at]cms.titech.ac.jp
Contact
*Please replace "[at]" with "@"
About Tokyo Institute of Technology
Tokyo Institute of Technology stands at the forefront of research and higher education as the leading university for science and technology in Japan. Tokyo Tech researchers excel in a variety of fields, such as material science, biology, computer science and physics. Founded in 1881, Tokyo Tech has grown to host 10,000 undergraduate and graduate students who become principled leaders of their fields and some of the most sought-after scientists and engineers at top companies. Embodying the Japanese philosophy of "monotsukuri," meaning technical ingenuity and innovation, the Tokyo Tech community strives to make significant contributions to society through high-impact research.
Website: https://www.titech.ac.jp/english/
About High Energy Accelerator Research Organization (KEK)
KEK was established to promote various types of researches as a center of excellence for overall development of Japan's accelerator science (particle and nuclear research using high
energy accelerators, research on the structure/function of matter including living organisms, research on improving the accelerator performance, and related basic technologies). As the Inter-University Research Institute Corporation, KEK provides researchers across the country and abroad with opportunities for research. With the Tsukuba and Tokai campuses as centers for excellence, KEK joins international collaboration experiments and developments.
In addition, KEK is in charge of the School of High Energy Accelerator Science of the Graduate University for Advanced Studies (SOKENDAI).
https://www.kek.jp/en/
About Japan Proton Accelerator Research Complex (J-PARC)
J-PARC is a high intensity proton accelerator facility at the Tokai campus, and aims at the frontier in materials and life sciences, and nuclear and particle physics.
It is a joint project between KEK and Japan Atomic Energy Agency.
SuperHRPD is installed at the Materials and Life Science Experimental Facility (MLF) of J-PARC.
https://j-parc.jp/c/en/index.html